Calculate the Activation Energy for the Reaction 2nocl
View more similar questions or ask a new question. Calculate the equilibrium constant K c for the reaction.

Chem 103 Final Exam Flashcards Quizlet
21 10-2 B.

. 100 102 kJmol The Arrhenius equation relates the rate constant of a reaction to ______. K and 0175 Lmols at 490. Calculate the pH and concentrations of all species in a 00037M carbonic acid solution at equilibrium.
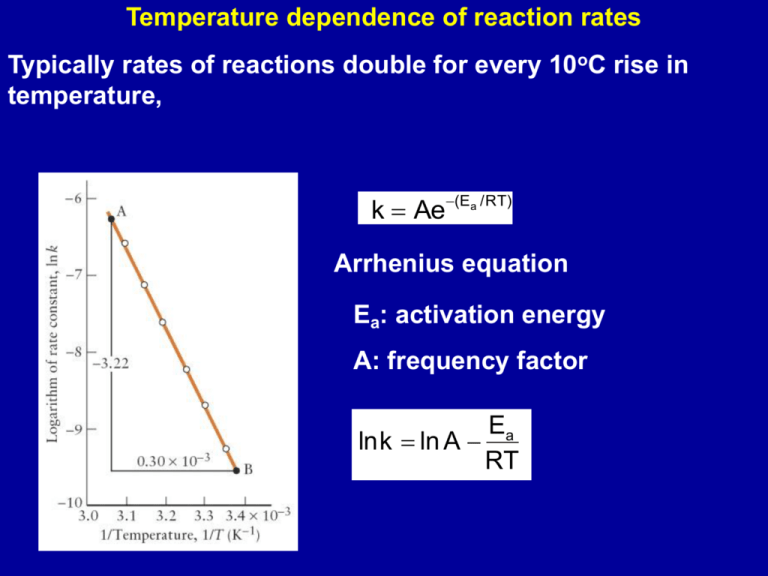
Students also viewed these Physical Chemistry questions. NO g Cl2 g - NOCl2 g NOCl2 g NO g - 2NOCl g Chemistry. Lnk 2 k 1 E a R x 1T 1 - 1T 2 where E a the activation energy of the reaction in Jmol R the ideal gas constant 83145 JKmol T 1 and T 2 absolute temperatures in Kelvin k 1 and k 2 the reaction rate constants at T 1 and T 2.
The rate law for the reaction 2NO g Cl2 g - 2NOCl g is given by rate k NO Cl2 A mechanism involving the following steps has been proposed for the reaction. What is then the activation energy given T 25 degrees celsius. For the reaction 2NOClg2NOgCl 2 g calculate the standard equilibrium constant at 298K.
A 90 kJmol D 610 kJmol B 90 kJmol E 610 kJmol C 350 kJmol Ans. The rate law for the reaction is a rate kNH 4 NO 2 b rate kNH 42NO 2 2c rate kNH 4 NO 2 d rate kNH 4 2NO 2 2e None of the above 8. Equilibrium is established for the reaction 2Xs Yg 2Zg at 500K K c 100.
Activation energy for the reaction is 44KJmole. The activation energy for the reverse reaction is greater than the activation energy for the forward reaction. The half-lives were found to be 161 min at 199C and 125 min at 230C.
A catalyst is added and the reaction rate constant increases from 34L3mole-3s-1 to 400 L3mole-3s-1. NO reacts with chlorine in a gas phase reaction to form nitrosyl chloride NOCl. The reaction 2NOCl g 2NO g Cl 2 g has rate-constant values for the reaction of NOCl of 93 10 6 s at 350 K and 69 10 4 s at 400 K.
Calculate the activation energy for this reaction. 2NOClg 2NOg Cl 2 g. The reaction 2 N 2 O 5 4 NO 2 O 2 has an activation energy of 100 kJmol and an enthalpy of 23 kJmol.
Calculate the activation energy of the reaction 2NO g. The activation energy can be calculated from slope -EaR. The reaction 2NOCl g 2NO g Cl2 g has a rate-constant for the reaction of NOCl of 93x10-6s at 350 K.
What is the rate constant at 435 K. Express your answer to three significant figures. Determine graphically the activation energy for the reaction.
Sketch a potential energy diagram for this reaction and. Calculate the activation energy for the reaction 2NOCl g 2NO g Cl2 g if the rate constant k is equal to 0286 Lmols at 500. See picture for reaction.
K 10 M s 2128 - 100-1 -1 200 - 0 8. Calculate the activation energy for the reaction 2NOCl g 2NO g Cl2 g if the rate constant k is equal to 0286 Lmols at 500 K and 0175 Lmols at 490 K. Carbonic acid H 2CO 3 is a diprotic acid.
Energy for the reverse reaction. 17 10-3 C. Calculate Kp for the reaction 2NOClg 2NOg Cl2g at 400C if Kc at 400C for this reaction is 21 10-2.
-the activation energy -the frequency of collisions between particles. The activation energy of the gas-phase reaction 2NOCl g -- 2NO g Cl2 g is 979 kJ mol-1 and the change in the internal energy in the reaction is ΔE 690 kJ mol-1. Calculate the activation energy for the reaction.
KaCH3COOH 18 x 10 -5. 2 Bond breakage requires energy. Activation Energy 1 In order to form products bonds must be broken in the reactants.
The reaction C 4 H 10 C 2 H 6 C 2 H 4 has an activation energy E a of 350 kJmol and the E a of the reverse reaction is 260 kJmol. 206 X 10-4 C. 3 Molecules moving too slowly with too little kinetic energy dont react when they collide.
Ka 1 43 x 107 and Ka 2 56 x 1011. It should result in a linear graph. Using this data 2 NO g Cl2 g 2 NOCl g Kc 320 X 10-3 NO2 g NO g ½ O2 g Kc 393 calculate a value for Kc for the reaction NOCl g ½ O2 g NO2 g ½ Cl2 g A.
Estimate H in kJmol for the reaction as written above. Graph the Data in lnk vs. Nitrous oxide N2O decomposes at 600C according to the balanced equation 2N2O g 2N2 g O2 g A reaction mechanism involving three steps is.
After equilibrium was established it was found that 28 of the NOCl had dissociated according to the equation. The activation energy for the following first-order reaction is 102 kJmolN2O5g -- 2NO2g 12O2gThe value of the rate constant k is 135 x10-4 s-1 at 308K. Thus reaction rate should increase with an increase in temperature.
A 0021 B 0039 C 0169 D 26 E 47 Ans. 484 X 10-3 E. The reverse reaction is exothermic.
The activation energy can be determined using the equation. Ggibbs energy0 kJmol NO2. 2NOClg2NOgCl2g Calculate the.
At what temperature will the rate constant be increased to 500 x 10-4s-1. If the activation energy for the reactions is 1002x105 Jmole what is the value of the rate constant at 475x102 K. The reaction 2NOClg 2NOg Cl2g has rate-constant values The reaction 2NOClg 2NOg Cl2g has rate-constant values for the reaction of NOCl of 93 10 6s at 350 K and 69 10 4s at 400 K.
If the activation energy is 104 kJmol calculate the temperature which its rate constant is 880 x 10-4 s-1 2 of 2 For the reaction NOg O3gNO2gO2g the frequency factor A is 87 x 10 12 s-1 and the activation energy is 63 kJmol. 25 mol NOClg was placed in a 250 L reaction vessel at 400 degrees C. Calculate the pH of a 032 M CH3COONa solution.
Given that the values of ΔH o and ΔS o of the reaction at 298K are 772kJmol 1 and 122JKmol 1 Medium Solution Verified by Toppr ΔH o772kJmol1000JkJ77200Jmol Using the relation ΔG oΔH oTΔS o ΔG o77200Jmol298K122JmolK ΔG o40844Jmol 1. Calculate activation energy for the reaction. Time give a straight line this reaction is second order with respect to NO 2 The rate constant k is the slope of the plot.
Variation of the rate constant with temperature for the first-order reaction 2N 2 O 5 g - 2N 2 O 4 g O 2 g is given in the following table.

Solved 6 At 100 C The Reaction Below Has A Rate Constant Chegg Com

Activation Energy Arrhenius Law Energy Activities Physical Chemistry Energy

For The Reaction 2nocl G Harr 2no G Cl 2 G The Equilibrium Constant Is 2 8xx10 5 At Youtube

Activation Energy Calculator Energy Calculator Energy Activities Energy

Solved 1 For The Following Reaction 2no G Cl2 G Chegg Com
136 Suppose The Activation Energy Of A Certain Reaction Is 250 Kjmol If The Rate Course Hero

Sample Test Questions General And Organic Chemistry Ch 313n Docsity
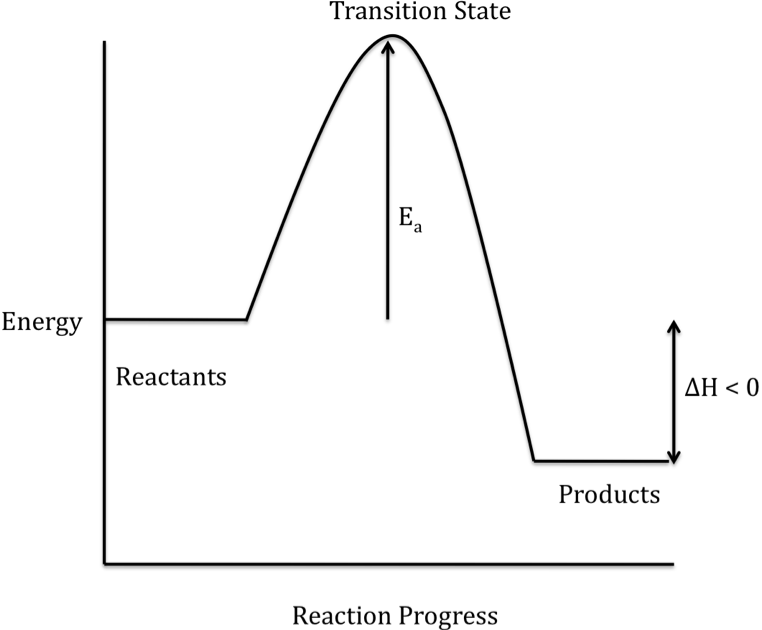
Top Of Page Periodic Table Andover S Chem 550 580 Advanced Chemistry Table Of Contents Chapter 19 Chemical Kinetics Section 19 1 Collision Theory And Factors That Increase Chemical Reaction Rates Section 19 2 Reaction Energy Profiles
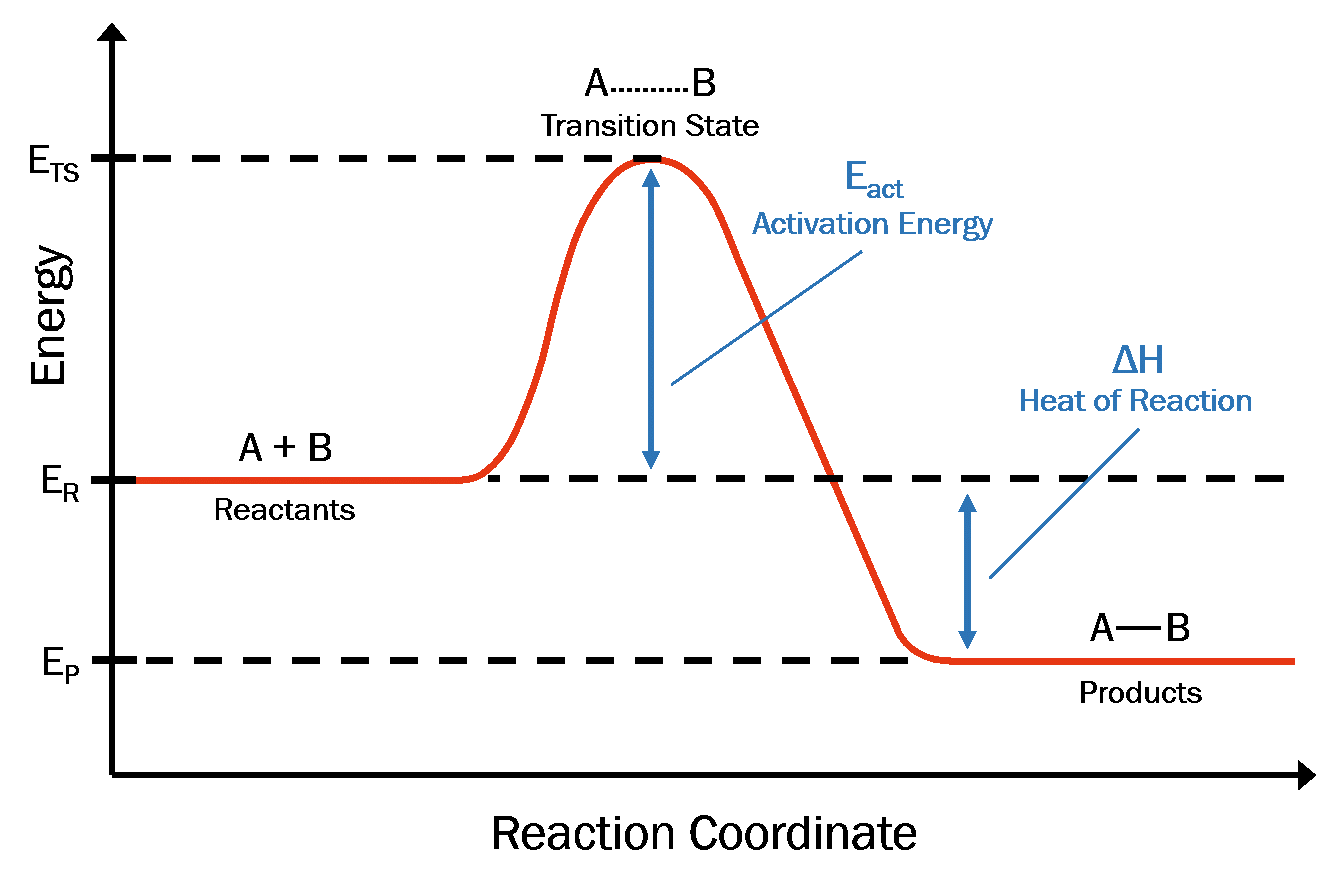
Rate Of Reaction Chemistry Quizizz
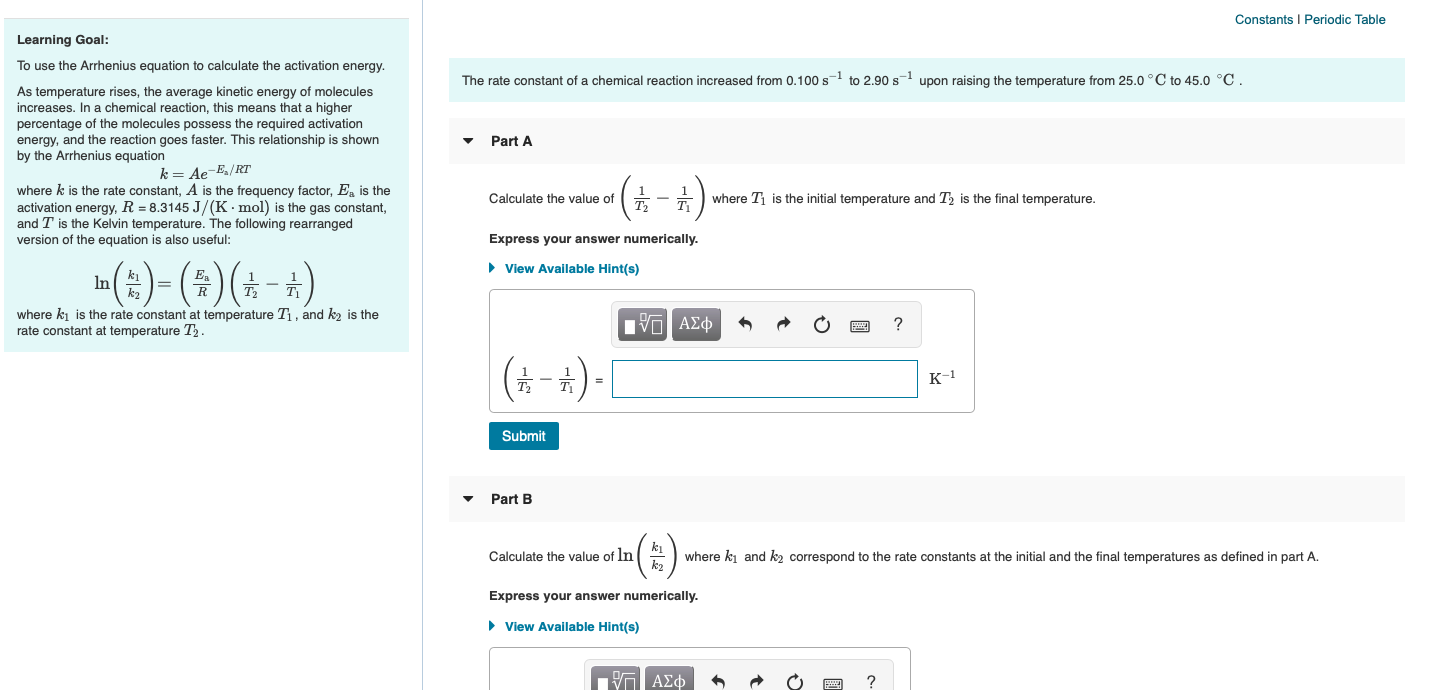
Solved Constants Periodic Table Learning Goal To Use The Chegg Com

Activation Energy Energy Activities Chemistry What Is Science

The Activation Energy Of A Reaction Is 94 14 Kj Mole And The Value Of Rate Constant At 313 K Is 1 8 10 1sec 1 Calculate The Frequency Factor A

Calculate Activation Energy Slope Or Formula Youtube
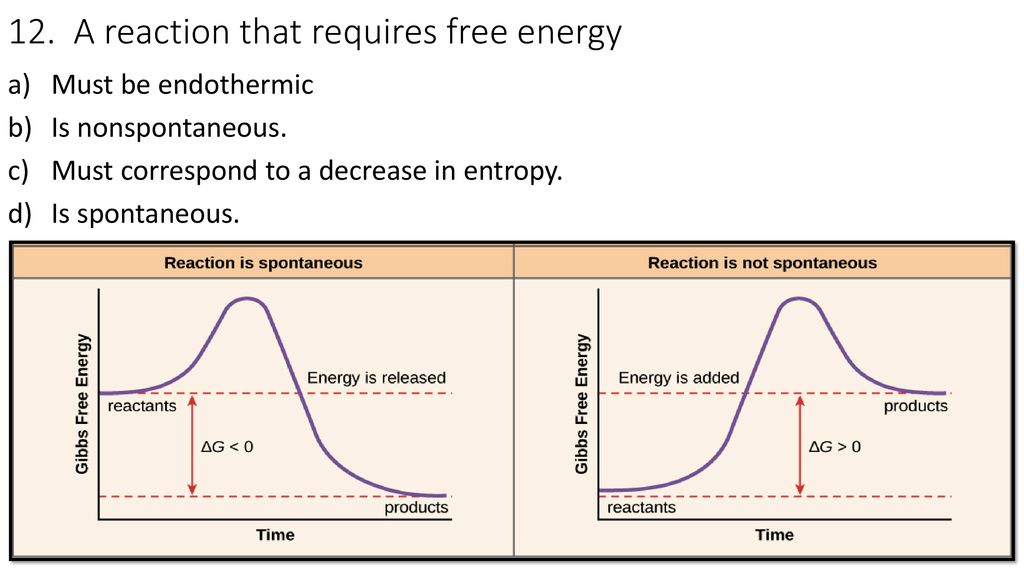
Matching I Activated Complex E Chemical Equilibrium A Reaction Rate H Entropy J Le Chatelier S Principle D Activation Energy C Spontaneous Ppt Download

Matching I Activated Complex E Chemical Equilibrium A Reaction Rate H Entropy J Le Chatelier S Principle D Activation Energy C Spontaneous Ppt Download
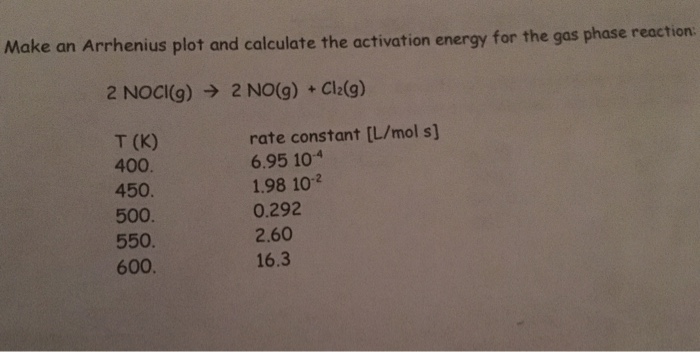
Solved Make An Arrhenius Plot And Calculate The Activation Chegg Com

Comments
Post a Comment